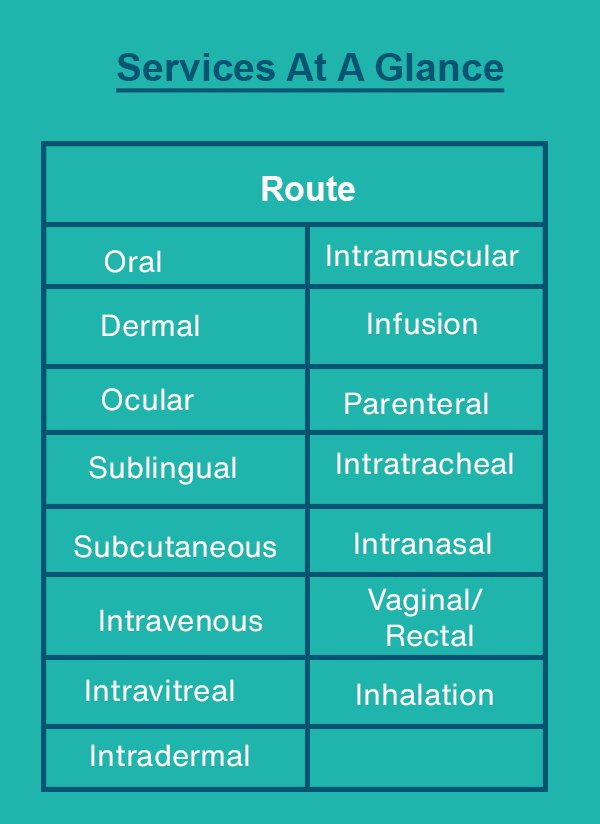
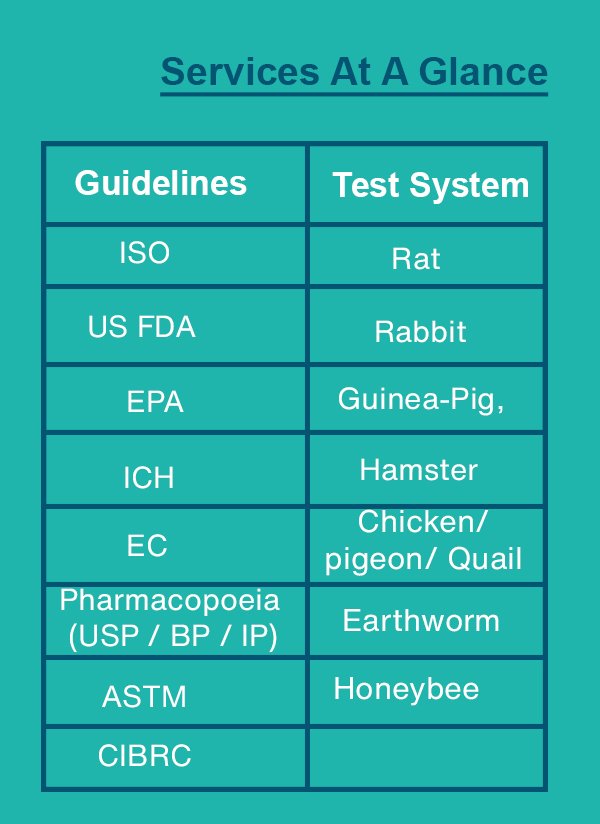
Biocompatibility Testing of Medical Devices
Crystal Biological solutions is committed to quality of work for medical devices for its biocompatibility as per ISO, US FDA guidelines for regulatory approvals. Test for in vitro Cytotoxicity (ISO 10993-5)
Toxicology
Toxicology studies are carried out as per various regulatory guidelines such as OECD, US FDA, ISO and Schedule Y guidelines.
- Maximum Tolerable Dose(MTD)
- Dose Range Finding (DRF)
- Acute Toxicity studies
Drug Testing
We have all equipped facility for different assay
Bioassays
- Erythropoietin bioassay – EP
- Insulin Biopotency Test (USP)
- Insulin Bio Identity Test (USP)
Agrochemical
We provide Toxicology Tests as per requirements of global regulatory .
In vivo Toxicology Studies
- Acute Oral Toxicity
- Acute Dermal Toxicity
- Acute Eye Irritation
- Acute Dermal Irritation
Microbiology
Our microbiology team is competent and trained to handle the products and to evaluate the presence of microbes under controlled conditions.
Microbiology Services
- Detection of Microbial Load
- Sterility
Research projects
Crystal biological solutions is having expertise in designing protocols to prototype for your research projects. Also we provide free consultancy to nurture ideas of innovators in the field of drug development and preclinical research.
Histopathology and Clinical Pathology
Our Histopathology & Clinical Pathology department is well equipped with quality instrument.
- Sample Collection and Preservation
- Sample Processing,

